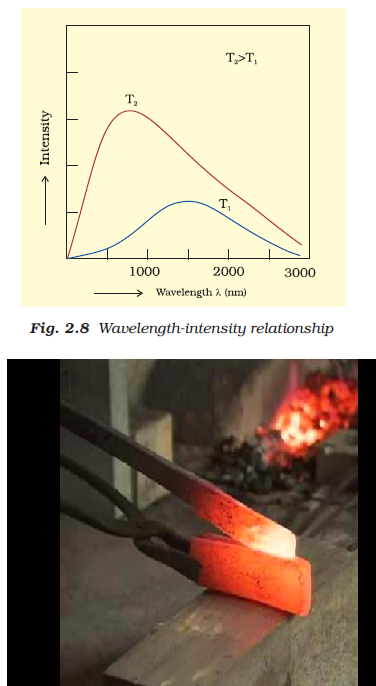
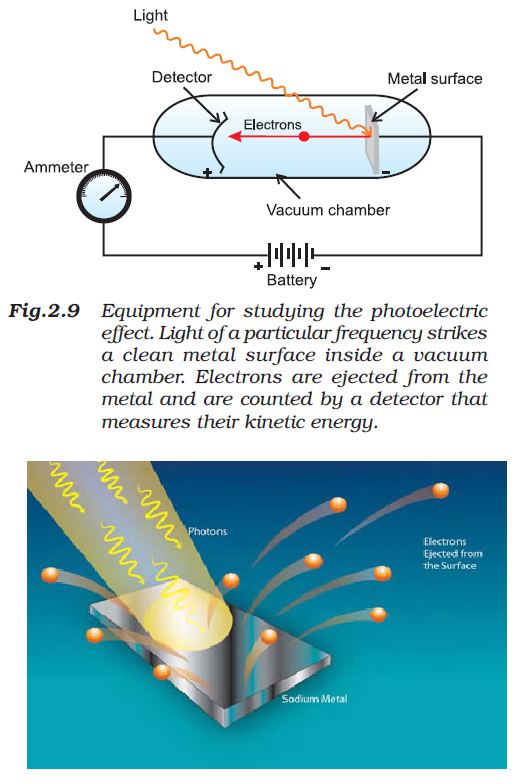
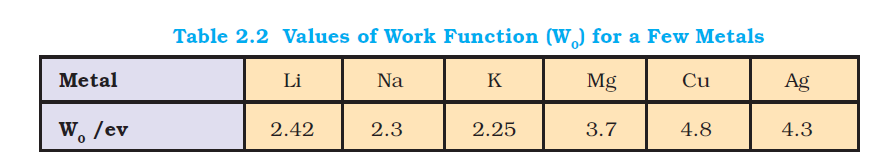
Characteristics of Electromagnetic Radiations :
(i) All electromagnetic radiations travel with velocity of light.
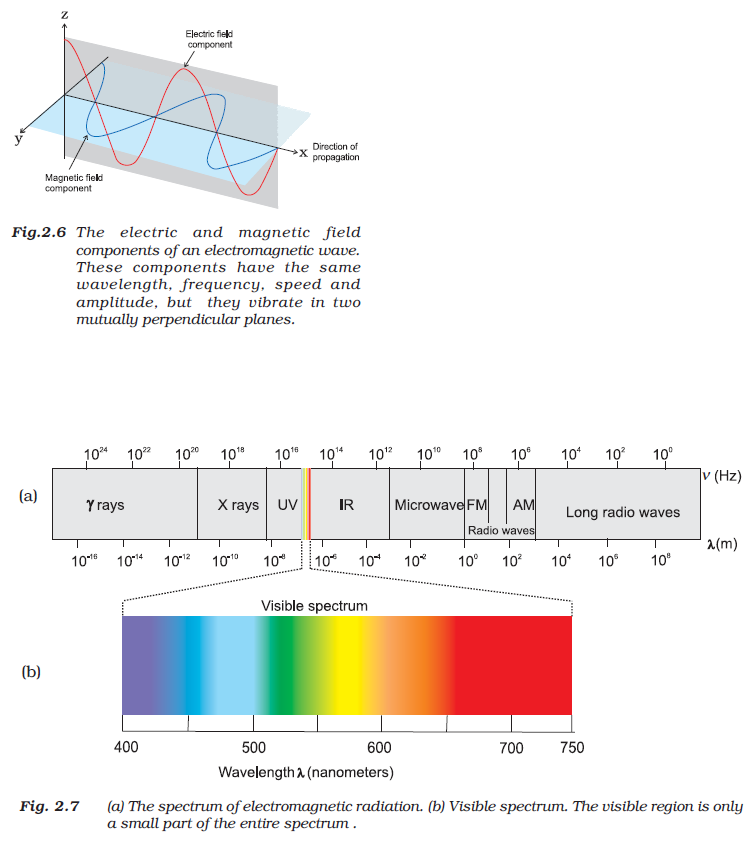
(ii) The oscillating electric and magnetic fields are perpendicular to each other and both are perpendicular to the direction in which the wave is travelling(Fig2.6).
(iii) According to Maxwell theory, energy of EM waves is proportional to the square of amplitude.
(iv) Unlike sound waves or water waves, electromagnetic waves do not require medium and can move in vacuum.
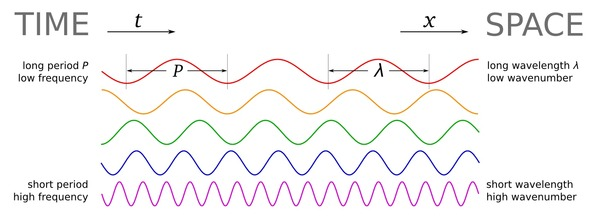
(v) There are many types of EM radiations which differ from one another in wavelength. This is called EM spectrum(Fig2.7).
(ii) The oscillating electric and magnetic fields are perpendicular to each other and both are perpendicular to the direction in which the wave is travelling(Fig2.6).
(iii) According to Maxwell theory, energy of EM waves is proportional to the square of amplitude.
(iv) Unlike sound waves or water waves, electromagnetic waves do not require medium and can move in vacuum.
(v) There are many types of EM radiations which differ from one another in wavelength. This is called EM spectrum(Fig2.7).